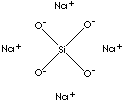
Sodium silicate, also called water glass or Soluble Glass, is any one of several compounds containing sodium oxide, Na2O, and silica, Si2O, or a mixture of sodium silicates varying ratios of SiO2 to Na2O, solids contents, and viscosity. Traditionally, sodium silicates are classified according to the acid from which they are derived as Orthosilicate Na4SiO4; Metasilicate Na2SiO3; Disilicate Na2Si2O5; Tetrasilicate Na2Si4O9. They are also classified by an X-ray diffraction method according to their crystalline structure. All these compounds are colorless, transparent, glasslike substance available commercially as a powder or as a transparent, viscous solution in water. They can be dissolved in water to form a syrupy liquid. Some forms are slightly soluble, and some are almost insoluble; they are best dissolved by heating with water under pressure. The solutions are strongly alkaline. They are produced chiefly by fusing sand and sodium carbonate in various proportions. The use of sodium silicates is as a raw material for making silica gel. Also used in detergent; as a cement for glass, pottery, and stoneware; for fireproofing paper, wood, cement, and other substances; for fixing pigments in paintings and cloth printing; and for preserving eggs.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||